CAIRECast DeepDive
Incident 33 Discussion
CAIRECast DeepDive is brought to you by NotebookLLM
Incident 33 – Vent battery drawn to MRI
Findings of the CAIRE Expert Panel Review
Background
During a daytime MRI procedure at a general hospital, a Maquet Servo MRI conditional respiratory ventilator was positioned at the designated gauss line before the patient was placed on the table. The MRI scanner in use was a 1.5T Siemens Espree. The incident was reported second-hand by a technologist.
After several minutes of scanning, therapists noticed an issue with the patient’s vital signs, which led to the termination of the examination. Upon entering the room to remove the patient, the battery pack from the ventilator was inadvertently pulled onto the magnet beneath the table. No injuries were caused by this device malfunction.
The facility did not use any handheld ferromagnetic detection (FMD) or metal detection devices, but it did have a Metrasens Ferroguard® wall-mounted FMD. Additionally, no Zone 4 barriers were used at the scanner room entry point.
Incident Review
The CAIRE Expert Panel reviewed this incident and had a number of questions about the event. These included wanting to better understand the MR Conditional conditions of the vent and its battery pack and the workflow and personnel training associated with this incident.
Even with the desire for additional information, the CAIRE Expert Panel was able to identify.
It is unclear what the MR Conditional conditions of the vent are, or how the static magnetic field condition(s) were expected to be met.
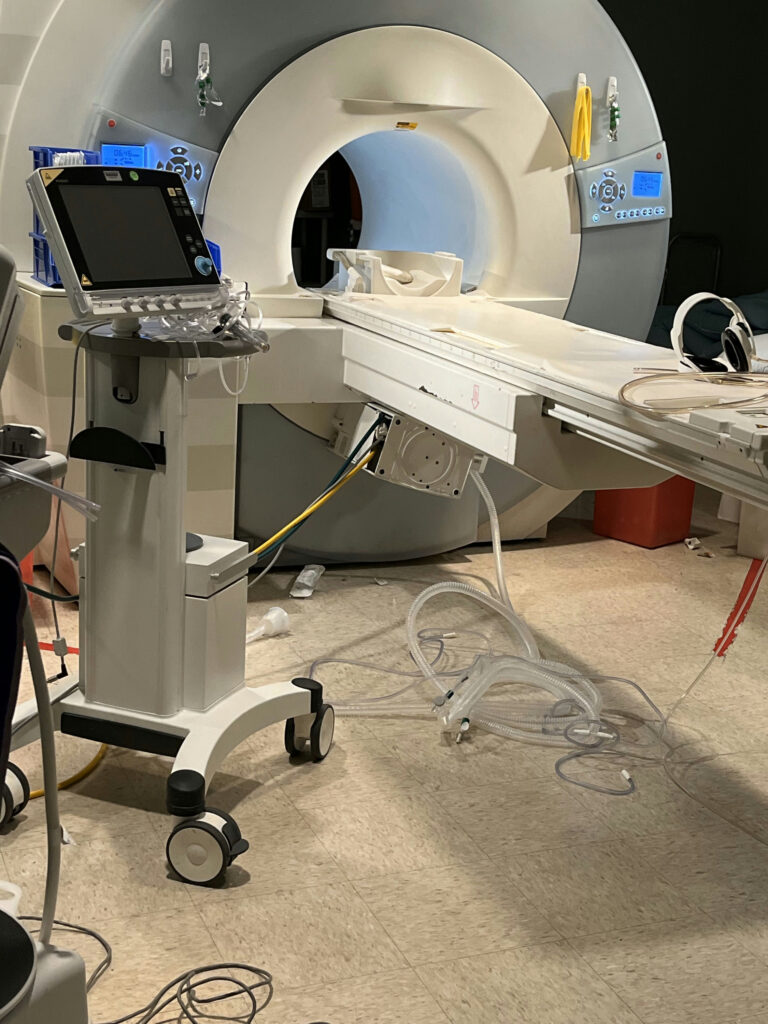
From the provided photograph, it’s unclear if the vent was labeled with the MR Conditional conditions (or if it was, was the labeling conspicuous enough to all who might be interacting with it from different directions). The photograph, as provided, does not show either a tether (to limit the proximity of the vent to the MRI scanner) or floor markings to indicate a designated ‘parking spot.’ Operational protocols for the proper use of location-restricted MR Conditional devices are important, but physical indicators (such as floor markings) or limiting functions (such as tethers) are very important real-time indicators of what the operational protocol is supposed to be.
-
Unclear if the vent was labeled with the MR Conditional conditions, or labeling was too conspicuous
-
No tether (to limit the proximity of the vent to the MRI scanner) or floor markings to indicate a designated ‘parking spot’ or gauss limits
-
Ability to immobilize the vent
-
Was the battery mount/retainer defective or was it inappropriately used by the site staff to affix the battery
-
Did the vent have inappropriate replacement components added when serviced, which would have increased the magnetic attraction risk of the device, or changed the device labeling?
Expert panel event questions based
on Incident 33’s provided image
⭐ ⭐ ⭐
The patient’s clinical decline appears to have contributed to a loss of focus on the MR Conditional vent.
It is essential for MRI providers, especially those who support imaging of high-acuity patients, to recognize and prepare for patient decline during MRI. Assuming that the vent was unadulterated (not inappropriately modified in a way that made it at greater risk of a projectile incident), the basic conditions of safe use would appear to have not been followed. The fact that the attracted battery wasn’t realized ‘in the moment’ is further indication for the Expert Panel that focus and attention was drawn away from adhering to the conditions of safe use of the vent to the decline of the patient.
The CAIRE Expert Panel had multiple questions about the vent device, its design, and servicing.
The CAIRE Expert Panel was concerned about the design and labeling of the vent in question, beyond the conspicuousness of MR Conditional labeling, expressed above. These questions included questions about the ability of the vent to be immobilized (e.g., wheel locks), whether the battery mount / retainer was effective (or was inappropriately used by the site staff to affix the battery), whether the vent was inappropriately serviced and had substitute / replacement components that increased the magnetic attraction risk of the device assembly.
The CAIRE Expert Panel had multiple questions about the training and protocols for use of the vent device.
The CAIRE Expert Panel was interested to know more about the specific training and operational protocols for the use of the vent in high-acuity patients. Did training and protocols effectively identify an individual who was principally responsible for the vent’s position and safety? Did the training for the staff include simulation training for a vented patient who needed rapid removal from the MRI? Assuming that the vent, itself, was not adulterated, the Panel felt that both team training and making sure that it was a designated individual’s duty to attend to the conditionality of the vent could have provided significant mitigation in this event.
Accreditation
This site had both Joint Commission enterprise-level accreditation and ACR modality-level accreditation, the MRI safety standards of which the Expert Panel felt were insufficient to have effectively prevented this incident.
Enterprise | The Joint Comission
While the The Joint Commission (TJC) is one of the accreditation organizations with the greatest number of MRI-specific standards, they are not specific to minimum practices at the point of care. As an example, TJC Diagnostic Imaging standards require the provider ‘manages MRI safety risks,’ but broadly does not identify what those risks are, or what specific practices are effective at managing the risks. TJC also requires MRI technologist training relative to which equipment is safe for use in MRI, but doesn’t identify what the safe use of that equipment process either includes or excludes. It is also worth noting that TJC only requires MRI safety training for MRI scanning technologists, and personnel typically present (and necessary) for MRI imaging of higher acuity patients, personnel such as nurses or respiratory techs, are not required by TJC to have any MRI safety training.
Modality | American College of Radiology
For the American College of Radiology (ACR) MRI accreditation programs it is actually rather difficult to identify what the MRI safety requirements are. Unlike several other accreditation organizations, the ACR appears to scatter its requirements across multiple different documents, on multiple different webpages. Unlike the rigorous, blinded independent review of image quality that is the hallmark of ACR MRI accreditation, MRI safety is largely subject to verification by the site’s retained medical physicist. The ACR’s MRI Safety Program Assessment Checklist only requires the existence of policies. Pertinent to this event, those policies that are expected to exist are the ones that speak to “documented MR safety education / training for all personnel,” “device and object screening,” and “designation of MR safe / MR conditional status.” The ACR’s requirements stop and the existence of policies. They offer no substantive or qualitative guidance on what those policies should contain, and they don’t require any minimum procedures or actions at the point of care.
It is worth noting that none of the other accreditation organizations, neither enterprise-level (such as TJC) nor modality-level (such as ACR), have the level of detail / specificity in their MRI accreditation criteria that the Expert Panel wishes to see. It is also important to note that the absence of accreditation standards for MRI safety is not because of an absence of clearly defined MRI safety practices. TJC, themselves, ‘retired’ their 2008 Sentinel Event Alert on MRI accidents and injuries (SEA #38) in favor of the published guidance on MRI safety practices from the ACR. The ACR publications on MRI safety, including the most recent 2024 ACR Manual on MR Safety, are widely regarded as the world’s leading collection of MRI safety practices.
The following criteria appear to have played a contributing role in this incident:
- The provider type (hospital providing MRI care to high-acuity patients)
- The vent (possibly adulterated, MR Conditional conditions not identified)
- Staff training (MR Conditional conditions not followed)
- Ineffective accreditation guidance / oversight
Recommendations from the CAIRE Expert Panel:
Sites should make MR Conditional status and conditions conspicuously evident on medical equipment.
The CAIRE Expert Panel recommends both conspicuous signage / coloration to be able to differentiate MR Conditional equipment from a distance, as well as apparent and accessible permanent signage which indicates specific limiting conditions of safe use. Examples of the first strategy (conspicuousness at a distance) might be a pennant flag on a mast attached to MR conditional devices, or conspicuous & different paint colors, or a ‘table tent’ sign affixed to the top of the vent. Examples of the second strategy could include laminated large-print conditions affixed to the device.
“Time out” procedure, in which equipment is inspected and conditions validated, conducted prior to usage.
In the parlance of the 2024 ACR Manual on MR Safety, a “full stop / final check” or “time out” procedure should be conducted prior to any equipment or personnel entering the MRI scanner room. This process should verify the appropriateness of any / all equipment being brought into Zone 4 (the MRI scanner room), clearly identify any limitations for usage (e.g., “wheels locked when parked beyond the 100 gauss line”), identify for all parties physical limits of safe usage (as applicable), and identify the device’s MRI safety ‘steward’ during this patient care episode.
Better means of indicating / maintaining distance from MRI in Zone 4 (MRI scanner room).
It is unclear from the report what indicators or limitations existed for the proper positioning of the device in Zone 4 (the MRI scanner room). It is recommended that when there are physical position limitations on the use of medical equipment within Zone 4, there ought to be clearly marked distance labels (based on the governing criteria for MR Conditional status) on the floor of the MRI room. To the extent that these boundaries can be labeled for their specific usage(s), that is preferable. The report also does not provide information on whether the vent cart was immobilized (e.g., wheel locks or tether) at a distance that would meet the MR Conditional conditions. The CAIRE Expert Panel recommends a formal process of ‘steward’ call-out and staff acknowledgement of securing the position of MR Conditional devices that have distance conditions.
Clarification of individual duty roles and drill / rehearsal of emergent situation.
When MR Conditional equipment with conditions which limit the positioning / proximity relative to the MRI scanner is used, members of the CAIRE Expert Panel recommended designating an individual ‘steward’ of the safe use of that equipment during the MRI examination. That individual would be responsible for verifying that the conditions will be met (limitations will be adhered to). The CAIRE Expert Panel also recommends having clear policies / procedures describing responsibilities and actions within Zone 4 (MRI scanner room) in emergent or “code” situations, and practicing code situations to help assure the integration of this practice information.
Accreditation organizations (both enterprise and MRI-specific) should make existing defined practices for MRI safety minimum performance criteria under the accreditation standards.
The CAIRE Expert Panel finds that existing enterprise and modality-specific accreditation standards do not identify MRI screening best practices, nor require their use at accredited providers. We recommend that accreditation organizations modify their MRI-specific standards to identify MRI safety best practices, and require the use of those practices for all entering the controlled access portions of MRI suites (Zones 3 and 4), including the use of equipment within the MRI scanner room.
Recommendations by:
Place
CAIRE Expert Panel recommends conspicuous physical labeling both equipment and the safe-use extents of the MRI scanner room (Zone 4). To the extent practical, the boundary condition labels should indicate the equipment / devices that the boundary is intended for. If a facility uses several pieces of equipment, each with different boundary condition values, it may be wise to consolidate floor markers to only one or two conservative boundaries. The panel also recommends the consideration of implementing tethers within Zone 4 to help prevent device encroachment beyond safety boundaries. Because of the risk of damaging RF shield assemblies, extreme caution should be taken when placing tether anchors in walls or floors of the MRI scanner room.
People
The CAIRE Expert Panel recommends training on the safe use of equipment within the MRI scanner room, and of the designation of a ‘steward’ of the use of MR Conditional devices within the MRI scanner room. We recommend code drills in order to rehearse the practical skills needed for safe operation within the MRI suite during urgent / emergent conditions.
Process
The CAIRE Expert Panel has several recommendations regarding process to help reduce the likelihood of similar incidents. Follow the ACR “full stop / final check” type “time out” process prior to entering Zone 4 (the MRI scanner room). Verify safety and conditionality of equipment to be used. Implement a call-out and acknowledgement process for securing MR Conditional equipment with location / proximity conditions, prior to proceeding with the patient positioning / exam.
Quick Links
Resources
- MRI Safety Docs
- CAIRE Manual
- ACR: MRI Safety
- MRI Safety Course
- FDA: MRI Safety

